Lipid Nanoparticles Encapsulation Project
- LNP formulation: ONPATTRO (patisiran)
- Lipid components: Dlin-MC3-DMA, DSPC, cholesterol, DMG-PEG 2000
- Lipid ratio: 50 : 10 : 38.5 : 1.5
- Flow rate ratio (Aqueous : Organic): 3 : 1
- N/P ratio: 3
DLS profile of LNP-siRNA at 12 ml/min flowrate
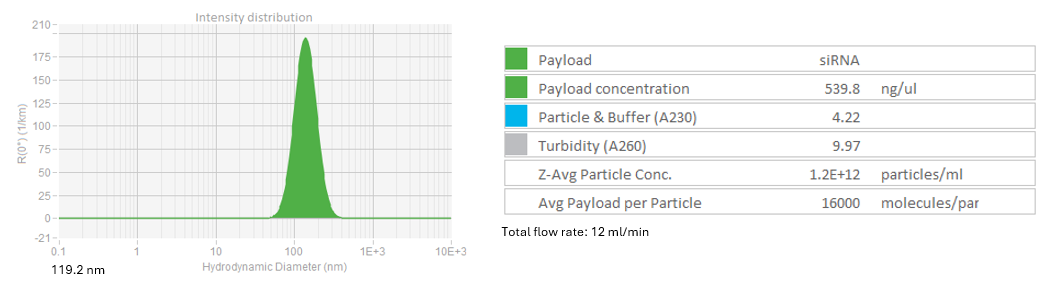
LNP-siRNA3: Initial RNA concentration 1.1 mg/ml
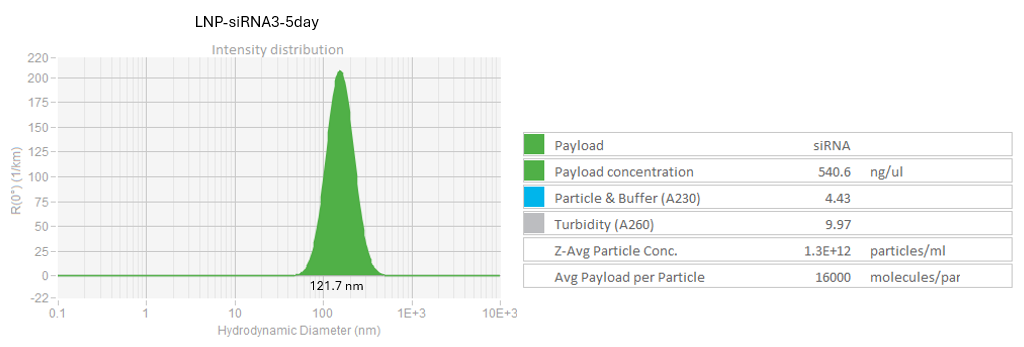
DLS profile of LNP-siRNA after 5 day storage at 4C
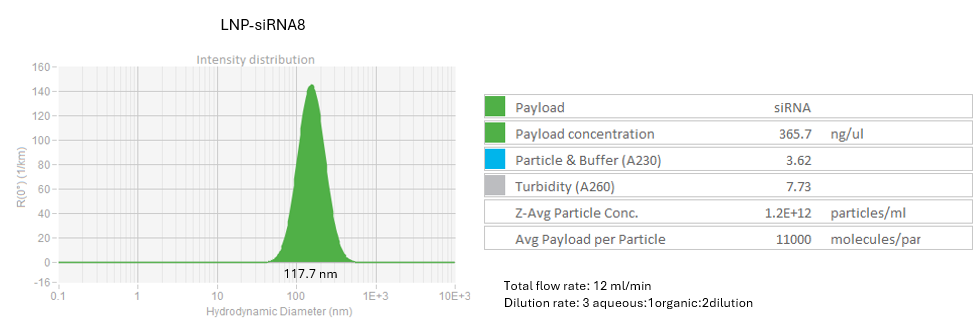
Testing dilution method
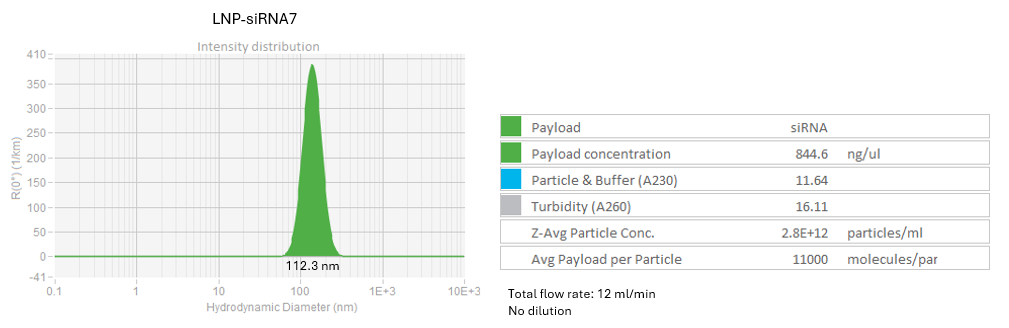
LNP-siRNA7, LNP-siRNA8: Initial RNA concentration 1.1 mg/ml
Dilute the LNP-siRNA using dilution buffer within Sunshine chip
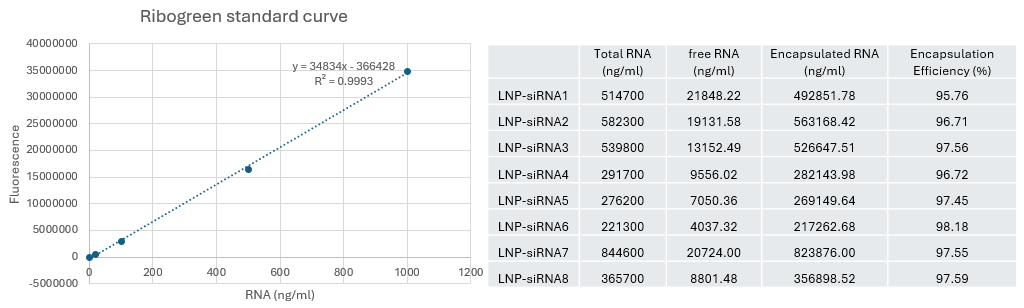
Encapsulation efficiency of several LNP-siRNAs we have tested in lab
Conclustion
- Flow rate at 12 ml/min is good to generate the LNP-siRNA with desirable size, ~120 nm
- Final concentration of LNP-siRNA is ~ 0.5 mg/ml when encapsulation siRNA solution at 1.1 mg/ml, with
aqueous : organic solvent 3:1 ratio
- LNP-siRNAs are stable at 4C for >5 days (still counting…)
- Encapsulation efficiency is very high at different flow rate when using Sunshine
Data demonstrating the use of Sunshine formulation parameters
to produce high-encapsulation, uniform LNP–siRNA particles with optimized N:P ratios,
low PDI, and strong gene-silencing performance.
You can insert full figures, tables, or captions here when ready.