With over 40 years of experience in oligonucleotide manufacturing, Bio-Synthesis supports diagnostic and testing professionals at every stage — from routine in-house assays to fully regulated kit production.
Our OligoDx™ platform delivers diagnostic-grade oligos with:
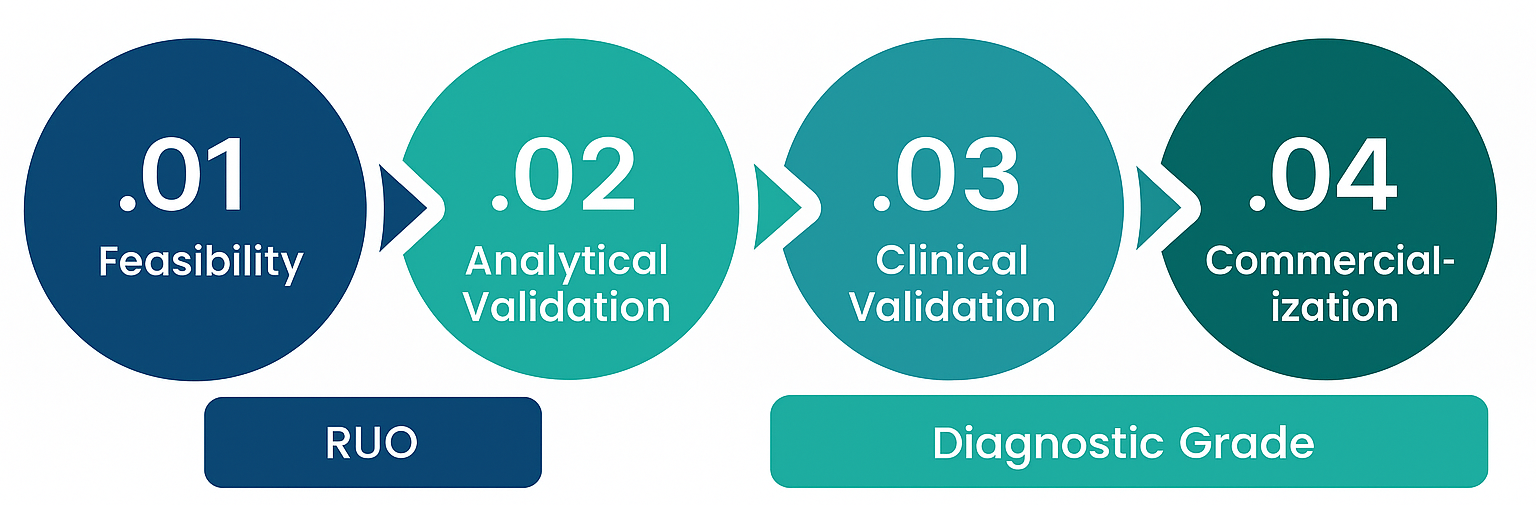
Let Bio-Synthesis accelerate your molecular diagnostic development — from feasibility through commercialization — with the reliability and precision your assays demand.
| Category | Feature | Research Grade | Diagnostic Grade |
|---|---|---|---|
| Process | |||
| Dedicated Account Contact Person | ✓ | Included | |
| Customized Fill & Finish | Option | Included | |
| Quality Management | |||
| ISO 9001 Certified Process | ✓ | ✓ | |
| ISO 13485 Certified Process | ✗ | ✓ | |
| Qualification / Validation (Equipment & Method) | Partial | ✓ | |
| Control | |||
| Quantification | Dual | Triple | |
| Stringent QC Tests (Validated) | ✗ | ✓ | |
| Traceability | Documented | Fully Documented | |
| Batch Record (Archived 5 Years) | ✗ | ✓ | |
| Classified Cleanrooms | ✗ | ✓ | |
| Certificate of Analysis (CoA) | ✗ | ✓ | |

Trusted by biotech leaders worldwide for over 40+ years of delivering high quality, fast and scalable synthetic biology solutions.