Since 1984, Bio-Synthesis has been a trusted partner to
scientists worldwide, providing high-quality, custom-synthesized
oligonucleotides for research, diagnostics, and therapeutic
development.
With decades of expertise in oligo production, we offer a
seamless path from early-stage research to full
commercialization.
Our team of scientific experts operates within state-of-the-art,
compliant facilities, ensuring that every product is delivered
with precision, consistency, and in full alignment with customer
specifications.
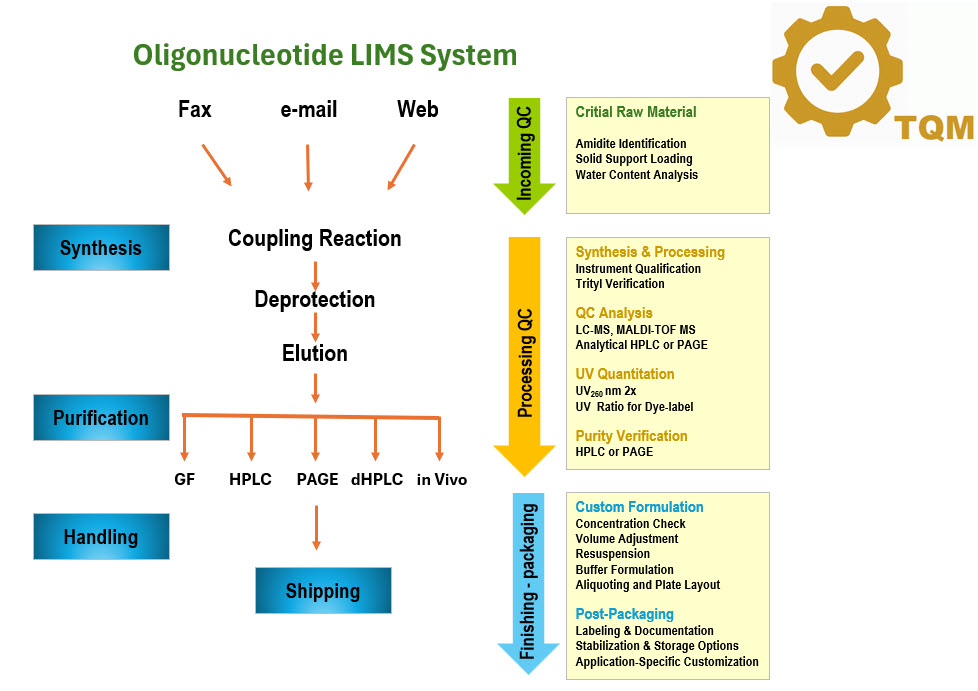
Delivering high-quality oligonucleotides starts with a robust and integrated total quality control and management system.
At Bio-Synthesis, our proprietary software seamlessly manages the entire process—from order entry and production tracking
to final product release and dispatch. This end-to-end system ensures that quality is monitored and maintained
at every step, providing our customers with confidence, consistency, and excellence from start to finish.
With over 40 years of expertise in oligonucleotide synthesis, Bio-Synthesis is uniquely equipped to handle even the most technically demanding custom biomolecules.
At Bio-Synthesis, our team of experienced Molecular Biologists and Chemists is here to support you at every stage — offering guidance in experimental design, application optimization, and technical troubleshooting.
When you work with us, you're not just getting a product — you're gaining a partner in scientific success.
At Bio-Synthesis, our unwavering commitment to quality is reflected in every oligonucleotide we produce. We follow stringent quality assurance protocols to ensure reliability, consistency, and performance:
With over four decades of manufacturing excellence, we stand behind our promise of high-quality, application-ready oligonucleotides. Learn more about our Custom Process Developments
To support your regulatory and research needs, Bio-Synthesis provides comprehensive quality control documentation, including:
Our quality documentation ensures transparency, traceability, and confidence in every batch delivered. Learn more about our Custom Release Testing and QC
At Bio-Synthesis, our our mid-scale oligo manufacturing process is designed to meet rigorous quality and regulatory standards. For all projects in the 1 to 1000 g range, we implement the following practices:
Our robust mid to large scale synthesis platform ensures high-quality, reproducible results—ready for downstream development.
Our state-of-the-art facility houses over 60 synthesizers, supporting the full spectrum of oligonucleotide production—from high-throughput small-scale plate synthesis to midscale and large-scale manufacturing—all under one roof.
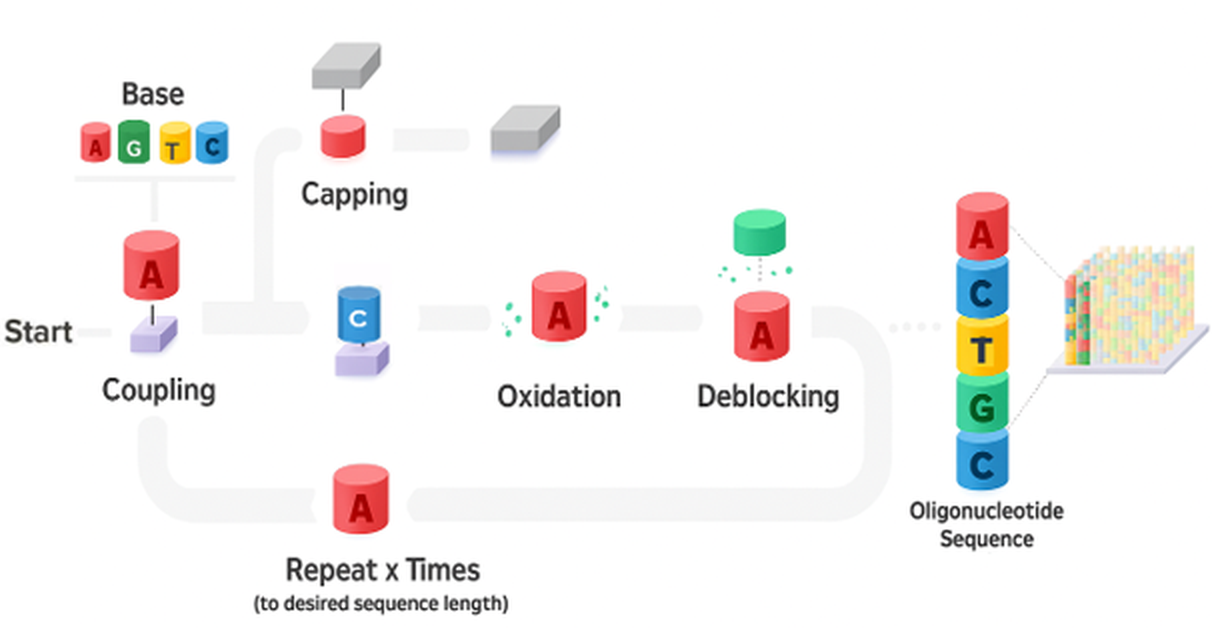




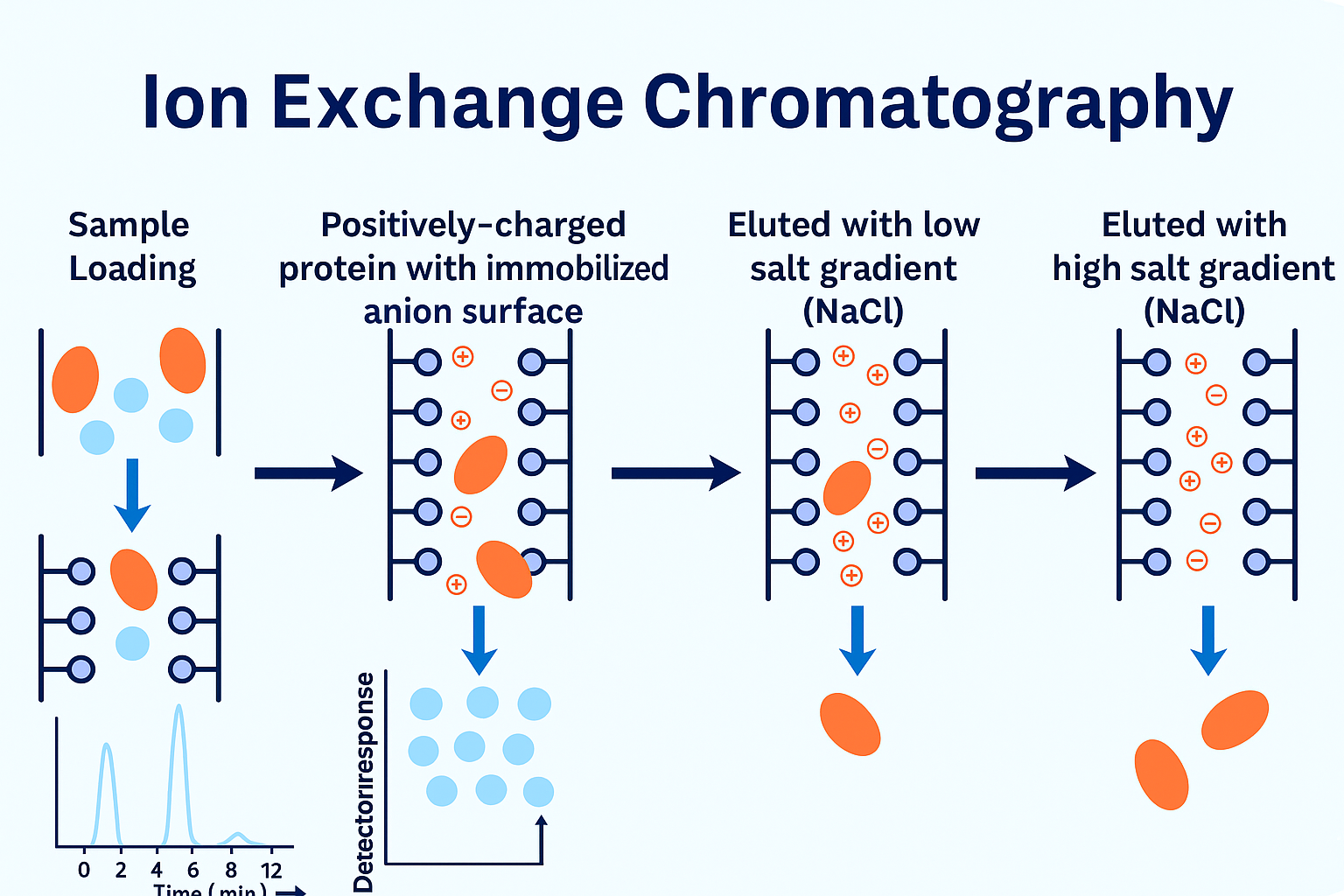
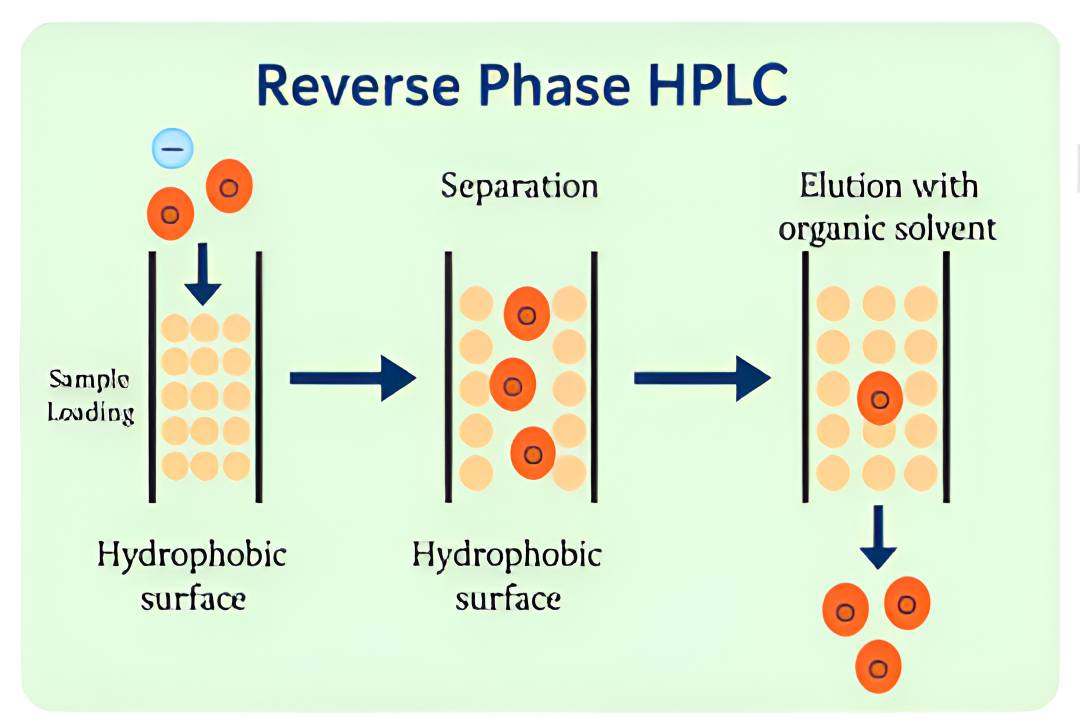
At Bio-Synthesis, purification is a critical component of our oligonucleotide manufacturing process—ensuring optimal yield and exceptional purity. Our experienced chromatographers and process chemists utilize advanced high-pressure liquid chromatography (HPLC) techniques to consistently deliver high-quality oligos. We provide end-to-end, scientist-to-scientist collaboration—from project planning to final delivery— supporting quantities ranging from micrograms to hundreds of grams. This commitment reflects our customer-first philosophy and dedication to excellence.
We offer industry-standard purification methods, including HPLC, reverse-phase, and ion-exchange chromatography. Additionally, our team can develop customized in-house protocols tailored to your specific yield and purity requirements.



Comming Soon.......

At the heart of our custom oligo solutions is an unwavering commitment to quality. From design to delivery, every step of our process is built to ensure precision, reliability, and performance—because your research deserves nothing less.
At Bio-Synthesis , we are dedicated to delivering excellence through the following core principles:
We strictly adhere to all applicable customer, quality, safety, and environmental requirements to ensure regulatory integrity and client trust.
We actively monitor satisfaction, respond to feedback, and implement improvements to consistently exceed expectations.
We drive continual improvement across our processes, people, and systems by setting and reviewing measurable objectives to enhance our Quality Management System (QMS).
Trusted by biotech leaders worldwide for over 40+ years of delivering high quality, fast and scalable synthetic biology solutions.